Jian Zhang
TU Dresden, Germany
Title: Efficient Hydrogen Production on MoNi4 Electrocatalysts with Fast Water Dissociation Kinetics
Biography
Biography: Jian Zhang
Abstract
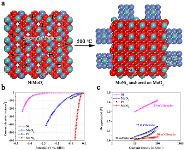
Large scale and sustainable production of hydrogen from water using the efficient and cost-effective electrocatalytic/photocatalytic/photoelectrocatalytic water splitting devices, e.g., water-alkali electrolyzers, is greatly promising for the future hydrogen economy. To this end, efficient, durable, and low-cost electrocatalysts are required to reduce the kinetic overpotentials of hydrogen evolution reaction (HER). Noble metal platinum (Pt) has been recognized as the most active and robust HER electrocatalyst with a near-zero onset overpotential and a high anodic current density. Unfortunately, the large-scale employment of Pt catalysts for hydrogen production is severely limited by its scarcity and high cost. For the HER in an alkaline solution, the kinetic process involves two steps: the prior water dissociation (Volmer step) and the subsequent combination of the adsorbed hydrogen (H*) into molecular hydrogen. Thus, once an electrocatalyst facilitates the initial water dissociation step on the surface, the HER performance will be improved. In this regard, we developed and design the novel electrocatalysts through engineering active sites for the water dissociation. For example, we demonstrate a novel out-diffusion strategy for synthesizing MoNi4 electrocatalysts, which can efficiently speed up the sluggish the Volmer step of the HER process in alkaline solution. The computational and experimental results reveal the fact that the kinetic energy barrier of the initial Volmer step is substantially reduced on the MoNi4 electrocatalysts. The as-constructed MoNi4 electrocatalysts supported by MoO2 cuboids exhibited an excellent electrocatalytic HER activity in 1 M KOH aqueous solution with an extremely low overpotential of ~15 mV at a current density of 10 mA cm-2 and a low Tafel slope of 30 mV decade-1, which are highly comparable to the results for the Pt and superior to those for state-of-the-art Pt-free electrocatalysts. Benefiting from its scalable preparation and excellent stability, the developed MoNi4 electrocatalyst is highly promising for practical water-alkali electrolyzer.