Atsushi Kitada
Kyoto University, Japan
Title: Electrochemical properties of AlCl3/glyme baths for room temperature aluminum electrodeposition
Biography
Biography: Atsushi Kitada
Abstract
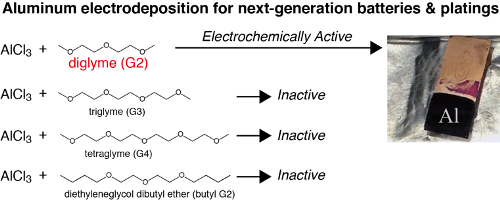
Aluminum (Al) metal, known for its low weight and corrosion resistance, finds its applications in structural materials. Smelting of aluminum has been industrialized as the Hall-Héroult process. Electrowinning of aluminum from alumina-dissolved cryolite molten salts is performed at 1000 °C. Instead of the high temperature process, medium and low temperature electrodeposition is fascinating as that can be hired as a new aluminum coating process. Several kinds of medium-low temperature baths for aluminum electroplating are reported using organic solvents (ethers, aromatic hydrocarbons, sulfones) and ionic liquids. Al electroplating is employed for the enhancement of corrosion resistance of magnesium alloys and steel. Additionally, the redox potential of aluminum is low and the theoretical capacity is high. Hence aluminum electroplating baths are captivating as a negative electrode material for the aluminum ion batteries. At electrodeposition temperatures, the usual organic solvents are highly volatile. Since ionic liquids are thermally and chemically stable and also less volatile at room temperature, they are adorable for electroplating baths. On comparing ionic liquids with organic solvents, however, cost of the chemicals required for the bath preparation is high for ionic liquids. Finding a cost effective and safe substitute for aluminum electroplating baths is important. Currently glymes, i.e. glycol ethers, have found to be relatively safe solvents for lithium and magnesium ion batteries, which have boiling points above 150 °C and relatively low volatilities at room temperature.The work reports on room temperature aluminum electrodeposition using aluminum chloride (AlCl3) and diglyme (G2), the first report for aluminum electroplating glyme baths. We also studied the electrochemical properties of AlCl3/glyme solutions using four kinds of glymes. By surprise, electrodeposition was only successful from G2 solutions: only G2 can form electrochemically active Al-Cl-glyme complex cations, which can then undergo easy desolvation of the glymes and subsequent reduction of Al3+.