Enrico Pizzutilo
Max-Planck Institute for Iron Research, Germany
Title: On-site production of hydrogen peroxide in electrocatalysis and in heterogeneous catalysis
Biography
Biography: Enrico Pizzutilo
Abstract
Hydrogen peroxide (H2O2) is an important green chemical oxidant, commonly used for disinfection, bleaching and water treatment. It is currently produced in large centralized reactors through the so-called antraquinone process. Considering that most of end-use applications require concentrations (around 2 to 5 wt%), an on-site continuous production of H2O2 at low concentration is desirable. Both the electrochemical reduction of oxygen (ORR) to hydrogen peroxide [1-2] and the direct synthesis in an autoclave [3-4] could provide a more efficient alternative to the current industrial process. Despite the state of the art characterization techniques, being applied to determine the surface structure of mono and bimetallic catalysts, there is still a lack of detailed understanding of the active sites of the metal nanoparticle and the actual reaction mechanism.
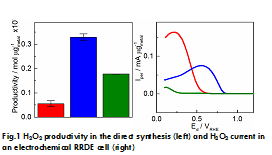
In this work different molar ratio of Au/Pd catalysts were synthesized [5] with a final metal loading of 10 wt% on activated carbon and the influence of bimetallic nanoparticles composition for Oxygen Reduction Reaction (ORR) and Hydrogen Oxydation Reaction (HOR) is studied. The change in activity, selectivity towards H2O2 as well as in H2O2 decomposition is characterized in both an electrochemical cell (electrochemical synthesis), using a Rotating Ring Disk Electrode (RRDE), and in an autoclave (heterogeneous direct synthesis). Whilst the addition of Au to Pd increases the overall selectivity, the results showed the lowest H2O2 productivity for pure Au during direct synthesis and the same catalyst showed the highest peroxide current in the electrochemical cell (Figure 1). This difference has been characterized and a mechanism for the H2O2 synthesis in the direct synthesis will be proposed.