Simon Geiger
Maxplank Institute for Iron Research, Germany
Title: Stability challenges of iridium-based oxides towards acidic water splitting – Structure matters!
Biography
Biography: Simon Geiger
Abstract
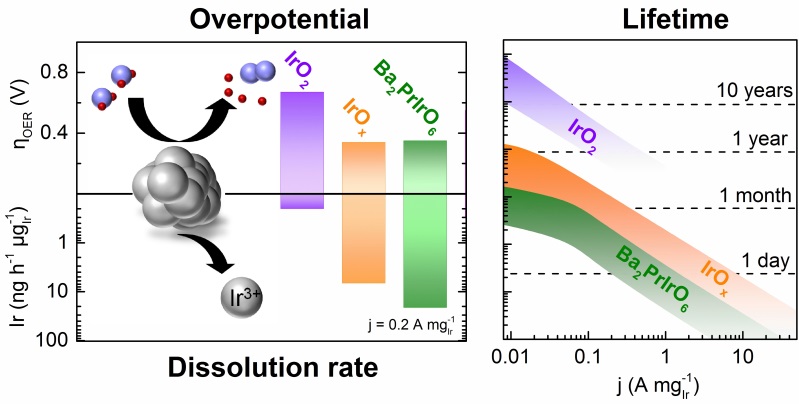
Reduction of noble metal loading and increase of specific activity are omnipresent challenges for oxygen evolution catalysts in proton exchange membrane (PEM) water electrolysis. The latter are often tackled by using iridium oxides with amorphous1or perovskite2,3structure with less focus on their stability during operation. In this presentation degradation processes of various iridium-based perovskites in relation to amorphous and crystalline iridium oxide are explored: Leaching of the non-noble elements in perovskites will lead to formation of amorphous iridium oxide, which is very active towards oxygen evolution but does not fulfil stability requirements4. Crystalline IrO2, on the other hand, resists the harsh operational OER conditions to a great extent, however, higher potentials have to be applied to reach the same current density. Combination of data on activity, dissolution, and structure are summarized in a conclusive dissolution pathway which is correlated with OER mechanisms. The instability of amorphous structures is explained by participation of activated oxygen atoms5, generating short-lived vacancies that favor dissolution. These insights are considered to guide further research which should be devoted to increasing utilization of pure crystalline iridium oxide, as it is the only known structure that guarantees lifetimes ≥10 years in acidic conditions. In case amorphous iridium oxides are used, solutions for stabilization are needed.