Junhua Dong
Professor
Title: Study on the galvanic corrosion of ferrite-pearlite steel and its control under an acid environment
Biography
Biography: Junhua Dong
Abstract
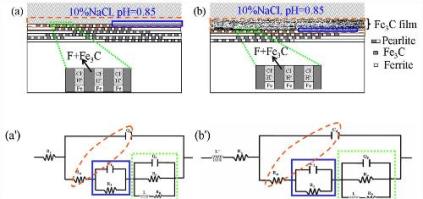
Statement of the Problem: Low carbon steel with the microstructures of ferrite and pearlite phases is the most widely used engineering structure material, in which pearlite with a typical lamellar structure consisting of alternating layers of ferrite and cementite (Fe3C) plays an important role in the mechanical properties of steel. However, as the electrically contact of Fe3C as a cathode site and ferrite site as anode in electrolyte can cause the galvanic corrosion, the corrosion resistance of steels will be rapidly deteriorated. The purpose of this study is to describe the sustained effect of the remaining cementite on enhancing the corrosion deterioration and the effect of several added alloy elements on retarding the corrosion of low carbon steel in an acid solution. Methodology & Theoretical Orientation: Immersion test, X-ray diffraction, micromorphology observation, potentio-dynamic polarization, open circuit potential monitoring and electrochemical impedance spectroscopy was utilized in this study. Findings: The results show that, with extending the immersion time, the preferential dissolution of the exposed ferrite resulted in a lot of cementite accumulation on the steel surface, which enhanced the galvanic effect and hence accelerated the corrosion. The promoted anodic dissolution of ferrite phases was controlled by charge transfer process, while the hydrogen evolution reduction occurred on cementite was controlled by the diffusion process. For 16MnCu steel, elemental Cu precipitated as the nano-sized particles on the steel surface, which improved the corrosion resistance of the steel by weakening the galvanic effect between the ferrite and cementite sites. Moreover, trace of alloy elements of Sn or Sn-Mo could improve the corrosion resistance of AH32 steel, which is also ascribed to that Sn or Sn-Mo could retard the galvanic corrosion between the ferrite and cementite phases. Sn and Mo should exist as the metallic state and were uniformly distributed on the steel surface.