Mitsuharu Tabuchi
Professor
Title: Synthesis and characterization of Ni- and Ti substituted Li2MnO3 positive electrode material for lithium-ion battery
Biography
Biography: Mitsuharu Tabuchi
Abstract
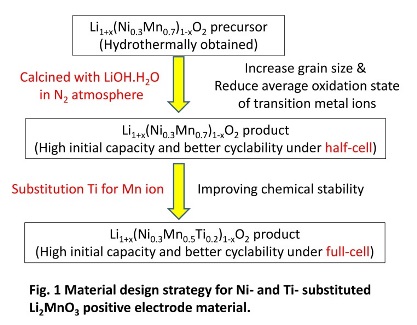
Li-excess positive electrode material (LiMO2-Li2MnO3 solid solution, M=Ni1/2Mn1/2 or Ni1/3Mn1/3Co1/3) is a good candidate as its high-capacity (250 mAh/g) and acceptable discharge voltage above 3.5 V for high energy-density lithium-ion battery to EV and PHEV application. Especially LiNi1/2Mn1/2O2-Li2MnO3 solid solution (Li1+x(NiyMn1-y)1-xO2, 0