Ingo Krossing
Professor
Title: The Protoelectric Potential Map – Unified Acidity and Reducity Scales and their Experimental Realization
Biography
Biography: Ingo Krossing
Abstract
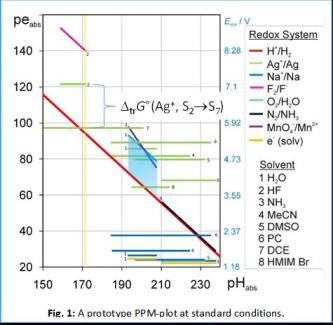
The comparison of acid-base and redox chemistry in their acidity (=> protonation) and reducity (=> electronation) scales is currently limited to measurements within one homogenous medium / solvent. Yet, it is crucial being able to measure chemical potentials of the proton and electron over medium boundaries to overcome this limit. Thermodynamically, the chemical potential differences of proton and electron are straightforward to describe, given the unified reference states ideal proton gas (1 bar) and electron gas (1 bar) we suggested in 2010 (proton gas)[1] and 2014 (electron gas).[2] This unifying concept was developed as part of our in March 2017 ceasing ERC UniChem project. The Protoelectric Potential Map PPM: In analogy to the proto- and electrochemical window of one medium like water, the PPM is a 2D plot of the medium independent pHabs vs. peabs values based on these unifying reference states. Any reaction with transfer of protons and / or electrons in any medium may be placed on the PPM. Given that the respective transfer energies are known , the thermodynamic relations between them may directly be compared over phase and medium boundaries.
This plot reveals at a glance the differences of reducity (i.e. electronation power, synonymous to redox potential) of a redox system (marked by colors) in dependence of the solvent S (marked by digits). For example, the Pearson soft deelectronator Ag+ (green) is up to about 3.5 V or 350 kJ mol–1 more effective in hard solvents like HF (2) or DCE (7) rather than in soft media like NH3 (3) or the Ionic Liquid (IL) HMIM Br (8).