M Lapkowski
Silesian University of Technology, Poland
Title: Electrochemistry of bipolar s-tetrazine derivatives
Biography
Biography: M Lapkowski
Abstract
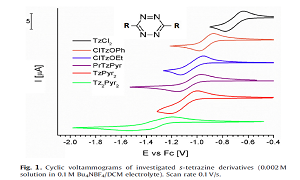
1,2,4,5-tetrazine, especially 3,6-disubstituted derivatives are very well known materials, in particular, in the field of the energetic chemistry. However, s-tetrazine ring has a number of other interesting properties. It is electroactive, with a very high electron affinity, furthermore it is highly colored. It is the smallest fluorophore, which makes s-tetrazine derivatives very promising molecules for active layers in optoelectronics devices such as organic light emitted diodes (OLED), electrofluorochromic and electrochromic windows. The functionalization of the ring with electron-donating group leads to obtain the donor-acceptor-donor (D-A-D) type of structure, which can serve as both: electron as well as hole transporting materials. Electrochemistry is a suitable method for characterization of new electroactive organic materials for optoelectronic and electronic applications. It provides lots of information about redox properties, stability, the conversion and storage of energy, etc. It also allows to determine the electron affinity and ionization energy of investigated compounds, parameters which are correlated with energies of HOMO and LUMO level, which need to be determined if materials are investigated towards optoelectronic applications. In this work we present the electrochemical and spectroelectrochemical characterization of bipolar s-tetrazine derivatives. The characterizations of studied compounds were performed using: electrochemical techniques including cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements; spectroelectrochemical investigations such as UV-Vis, EPR, Raman and fluorescence spectroelectrochemistry. The electrochemical characterization indicated that a few of studied s-tetrazine derivatives undergo electrochemical polymerization (oligomerization) which is rarely observed in this group of compounds. Hence, monomers as well as electrochemical obtained polymers were studied. Their redox processes have been investigated by in situ UV-Vis and EPR spectroscopy. A huge effect of chemical structure on the electrochemical properties was observed. Introduction of oxygen atom as a linker between donor and acceptor part of molecules resulted in obtained thin polymer layer with unique properties.