Elod L Gyenge
University of British Columbia, Canada
Title: Novel manganese dioxide-based electrocatalyst formulations for bifunctional oxygen reduction and evolution reaction activity
Biography
Biography: Elod L Gyenge
Abstract
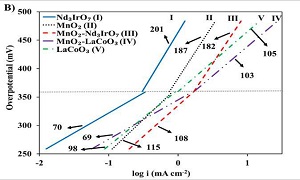
Development of highly active, durable and cost-efficient bifunctional electrocatalysts for the oxygen reduction and evolution reactions (ORR and OER) is of outmost importance for commercialization of rechargeable metal-air batteries (e.g., Zn-air, Al-air, Mg-air, Li-air) and regenerative H2-O2 fuel cells. Manganese dioxide (MnO2), a low cost and abundant material, has been intensely studied as ORR electrocatalyst in alkaline media. Regarding the bifunctional ORR and OER electrocatalytic performance, however, enhancement of the activity (e.g., lower surface overpotential at practical current densities above 100 mA cm-2) and improvement of the long-term stability are required for potential implementation in commercial systems. The purpose of this study is to present novel approaches for tuning the MnO2 performance with co-catalyst addition, potassium ion doping and support effect (e.g., graphene and graphitized carbon). The combination of MnO2 with structurally different oxide co-catalysts such as perovskite (LaCoO3) or fluorite-type oxide (Nd3IrO7) produces a synergistic catalytic effect improving the bifunctional (ORR and OER) activity compared to the individual oxides. Doping of the oxide catalyst with potassium ions, either by long-term exposure to 6 M KOH or potential driven insertion (PDI), increases further the activity and durability as revealed in accelerated degradation experiments. Optimizing the MnO2 electrodeposition conditions can produce nanostructured morphologies that are favorable for ORR and OER activity. The electrochemical studies are supported by extensive surface analysis (SEM, TEM, XPS, EDX, EELS). This work reveals new oxygen electrode catalyst formulations for rechargeable metal-air batteries and regenerative fuel cells.