Day 2 :
Keynote Forum
Joachim Maier
Max Planck Institute for Solid State Research, Germany
Keynote: The connection between chemistry and electric function in solids
Time : 09:30-10:00

Biography:
Joachim Maier studied Chemistry at the University of Saarbrücken, received his PhD in 1982 from the same university and completed his Habilitation at the University of Tübingen in 1988. He has lectured at the University of Tübingen, at Massachusetts Institute of Technology as a foreign Faculty Member, at the University of Graz as a Visiting Professor, and at the University of Stuttgart as an Honorary Professor. He is Past President of the International Society of Solid State Ionics. As Director of the Physical Chemistry Department (since 1991) of the Max Planck Institute for Solid State Research and Member of various national and international academies his concern is the conceptual understanding of chemical and electrochemical phenomena involving solids as well as their use in materials science. He has been listed as one of the most influential scientific minds (Thomson Reuters).
Abstract:
In loose terms chemistry is the chemistry of the perfect state (perfect crystallographic structure) plus chemistry of the excited state (defect structure). The latter is responsible for the electric transport and storage properties. In aqueous solutions this function is taken by H+ and OH- ions as well as dissolved ions. In solids this role is carried out by point defects such as excess (interstitials) and lacking particles (vacancies). It is exactly the consideration of point defect chemistry which is necessary to understand and tune ionic transport phenomena in solids hence forming the bridge between chemistry and electric function. This picture also comprises the electronic transport enabled by excess electrons and electron holes. It is shown how the charge carrier chemistry can be understood, analyzed and varied as a function of stoichiometry and doping not only in the bulk but also at interfaces. Of special interest are size effects on the electronic and ionic carrier concentrations. These defect-chemical considerations directly translate into the electric function in batteries, fuel cells and photo-electrochemical devices. This does not only hold at or near equilibrium, also the kinetic performance depend on such issues. In addition to transport-related questions, the point defects are most relevant acid-base or redox-active centers and are thus of central significance, not only for transport, but also for reaction kinetics and catalysis. A selection of applied examples such as storage modes in batteries, reaction kinetics in fuel cells or transport effect in photo-perovskites will be addressed.
Keynote Forum
Jelena Popovic
Max Planck Institute for Solid State Research, Germany
Keynote: Interfacial effects and charge carrier chemistry in lithium electrolytes
Time : 10:00-10:30

Biography:
Jelena Popovic is a Scientist at the Max Planck Institute for Solid State Research in Stuttgart, Germany since 2011. Her academic background includes a degree in Chemical Engineering from the University of Belgrade, Serbia in 2008 and a PhD in Colloid Chemistry from University of Potsdam/Max Planck Institute for Colloids and Interfaces in Potsdam, Germany in 2011. Her scientific interests range from new materials and transport mechanisms in ionic materials to soft matter electrochemistry and sustainable synthesis of nanomaterials.
Abstract:
Lithium electrolytes that link high ionic conductivities with high lithium transference number are rare, and believed to be essential for functional high power batteries. One effective way to prepare such materials is by engaging an interfacial effect on an oxide surface in order to demobilize the anion in liquid/solid electrolytes. The galvanostatic polarization experiments as well as the influence of surface area, salt concentration and temperature on their outcome will be discussed in details. Furthermore, significance of interfacial effects in other ionic devices will be touched upon. Rather than just facilitating high performance materials, liquid/solid electrolytes are a fruitful playground for fundamental understanding of the electrical double layer. A model glyme on muscovite mica system is a starting point for tackling the issue of ion-ion correlations in concentrated electrolytes and its effect on the Debye lengths estimated from the surface force measurements. Finally, solid polymer lithium electrolytes can be used in bilayer graphene gating experiments. Here, the electrolyte plays a vital role in the direct measurement of the high lithium diffusion coefficient.
- Theoretical and Computational Electrochemistry | Batteries and Energy Storage Sensors Physical and Analytical Electrochemistry | Photoelectrochemistry | Electrochemical Energy Electrochemical Engineering | Electrochemical Water Treatment | Electronic Materials and Processing Dielectric Science and Materials
Location: Olimpica 1

Chair
Guowei Li
Max Planck Institute for Chemical Physics of Solids, Germany

Co-Chair
Jelena Popovic
Max Planck Institute for Solid State Research, Germany
Session Introduction
César Pascual GarcÃa
Luxembourg Institute of Science and Technology, Luxembourg
Title: Electrochemical regulation of the acidity in miniaturized electrochemical cells: The route to increase flexibility and multiplexing of chemical control

Biography:
César Pascual García graduated in Solid State Physics from the Universidad Autónoma of Madrid in Spain with a dissertation of electronic optical transitions in III-V semiconductors. He obtained his PhD in Condensed Matter Physics in 2007 from the Scuola Normale Superiore of Pisa in Italy with the thesis: “Low lying excitations of few electrons quantum dots”. At the beginning of his career he collaborated in fundamental topics centered on the electron correlations of semi- and super- conductor materials, but then his interest shifted to biology-applied topics as he started working as Scientific Officer at the Institute for Health and Consumer Protection of the European Commission. Currently he is an ATTRACT fellow and Lead Research Scientist at the Luxembourgish Institute of Science and Technology where he leads the activities for electrochemical sensors and actuators for medicine applications at the Materials Research and Technology Department. His current research focus is semiconductor nanowires for bio-sensing and the miniaturized control of chemical reactions.
Abstract:
Better computer controlled systems to perform nanoscale chemical tasks is a demand for the fabrication of high-throughput microarrays and reprogrammable sensors dedicated to precision personalized medicine. The very limited tools that we have today to control chemical reactions in miniaturized devices is one of the main barriers for the control of massive multiplexing (>1 Mega spots). The proton concentration is one of the building blocks that could be used to control the kinetics of chemical reactions. Currently the multiplexed systems for high-yield in-situ synthesis of commercial microarrays are driven by optically triggered acid-labile groups. The electrochemical control of the proton release would be a natural way to control the acidity due to the high speed efficiency of redox processes, and would allow combining microarrays and programmable electrochemical sensors. However, only a couple of attempts can be found in literature to control chemical reactions in miniaturized environments by an electrochemically driven proton concentration. The limited surface to volume ratio of the electrodes and the fast diffusion of protons decreased the performance of these systems. Here we present our studies to control reversibility of redox processes that can be used to change the pH in microfluidic environments during many cycles, and a microfluidic design to control the fast diffusion of protons. With our system we show a pH swing comparable to the highest achieved by electrochemical systems of few millilitres, but in a device where the acid is confined in nano-litter volumes. The design promises high yield in-situ chemical synthesis since the system is compatible with lateral resolutions in the micron range assuring the stability of acid contrast between close spots.
Roy Zamora
Costa Rica Institute of Technology, Costa Rica
Title: New approach of flexible electrodes coated with carbon nanotubes/poly(3,4-ethylenedioxythiophene (PEDOT) for mancozeb analysis in water

Biography:
He has experience in polymers, by profession he is an Industrial Chemist, he has a Master's degree in Industrial Engineering, he is currently a PhD student and works at the Technological University of Costa Rica (TEC), as a Professor at the Materials School and works with the laboratory's chemical regent of polymers of the National Institute of Learning. His research area focuses on standardization of procedures, validation of methods and conducting and nano-structured polymers.
Abstract:
The extensive use of pesticides in crops generates a negative environmental impact affecting water quality and organisms. The intensive use of mancozeb pesticide (MCZ), in developing countries such as Costa Rica can cause severe chronic diseases in people. Therefore, it is paramount to access the residual amount of this agrochemical in water bodies. The purpose of this work is to develop a novel and economical electrode to detect mancozeb in water by electrochemical techniques. The electrodes of poly(3,4-ethylenedioxythiophene) (PEDOT) mixed with CNTs were characterized using thermogravimetric analysis (TGA), atomic force microscopy (AFM) techniques and its recovery after leaching through a sand column. Cyclic voltammetry was applied to characterize the electrochemical behavior of MCZ and its quantification in commercial formulations. The PEDOT/MWCNT electrode provides a robust electrochemical response in the linear range in addition to a faster procedure that can be conducted with fewer solvents and is more environmentally friendly compared to other techniques used to measure MCZ. Measures of this signal intensity as a function of concentration were used to quantify MCZ. Linearity yields a value over R>0.99 in the range from 25 to 150 μmol/L. The recovery value obtained for the tap water was 51.2 μmol/L equivalent to 102%. Speed on signal outputs and the feasible procedure make this new approach a candidate to undertake monitoring programs for ecological, agricultural and hydrological applications.
Essen N Suleimenov
Kazakh British Technical University, Kazakhstan
Title: The impact of M Faraday’s work on the development of natural science

Biography:
Essen N Suleimenov graduated from the Kazakh Mining and Metallurgy Institute, Metallurgy Faculty in 1960 with a specialty of Metallurgical Engineer in the area of non-ferrous, rare and precious metals. He is a Candidate of Technical Sciences (1970), Senior Research Associate (1981), Doctor of Technical Sciences (2005). He is a Fellow of the International Informatization Academy (2004) and Member of the European Academy of Natural Sciences (2007). After graduation he was assigned to work in the Institute of Metallurgy and Ore Beneficiation of the Academy of Sciences of Kazakh SSR. During his work in IMaOB he performed the job duties of a Senior Laboratory Technician (1960-1961); Engineer (1961-1963); Junior (1963-1971) and Senior (1972-1986, 1995-2000) Research Associates; Research Team (multidisciplinary) Leader (1985-1995); Head of Laboratory (2004-2005); Head of Department (2005-2006); Deputy Director for Science (2000-2004) and; Acting Director of the Institute of Metallurgy and Ore Beneficiation (2004).
Abstract:
The scientific heritage of M Faraday in the conditions of the modern crisis in natural science plays a decisive role in the development of ideas about the nature of the chemical bond and the practical use of chemical processes. In the 20th century, a huge experimental material was obtained, which confirms the correctness of M Faraday's views on the effect of electric current on chemical reactions. It was shown in that electrolysis occurs only in the places where the current flows. But electrochemistry has gone on the wrong path, which ultimately led to unjustifiably high losses of funds and labor in developing technologies for extracting metals from complex raw materials. In recent years, researchers have published an increasing number of materials aimed at establishing the principle of the formation of liquid systems and the determination of the mechanism of transport of an electric current through a liquid in the light of the theory of M Faraday. However, in the development of modern natural science the following basic thesis of the works of M Faraday plays: Identity of energy manifestations in the interaction of material objects and; the discrete nature of the electric current. The discrete nature of the electric current makes it possible to use a combination of electrical conditions for organizing unusual chemical reactions. The provision on the identity of energy manifestations of the interaction of material objects provides the basis for the revision of scientific provisions on the mechanism of heat exchange between material objects. Modern science has accumulated a huge amount of experimental material, including the unusual behavior of condensed systems under the influence of various energy effects, which gives grounds for revising the basic fundamental provisions of physical chemistry and theoretical inorganic chemistry.
Rajiv Prakash
Banaras Hindu University, India
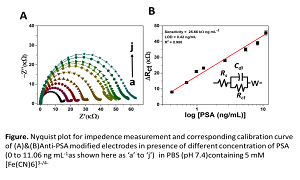
Title: Immunosensor for label-free PSA cancer detection on GQDs-AuNRs modified screen-printed electrodes

Biography:
Rajiv Prakash is a Professor and Coordinator of the School of Materials Science and Technology, Indian Institute of Technology, Banaras Hindu University, India. He has served as Scientist at CSIR lab Lucknow, India for more than 7 years before joining Indian Institute of Technology. He has been recipients of Young Scientist (Council of Science and Technology), Young Engineer Awards (INAE) of India and Materials Society Medal Award of India. His current research interests include synthesis of morphology controlled organic conducting polymers, nanocomposites, fabrication and characterization of organic electronic devices and sensors/biosensors. He is having more than 150 publications in international journals of repute and 17 patents in his credit. He is in Editorial Board of several National and International Journals. He is Member of various national committees including DST-TIFAC for India Vision 2035 and MHRD IMPRINT program.
Abstract:
Literature reveals that, in males, prostate cancer is ranked second as leading cause of death out of more than 200 different cancer types. Prostate specific antigen (PSA) is a 33-kDa serine protease, which is largely bound to endogenous protease inhibitors in human blood serum and its concentration in serum is used as indicator for prostate cancer. In healthy males the PSA concentration level ranges from 0 to 4 ng ml-1 in the serum. There are several PSA detection methods available like enzyme-linked immunosorbent assay (ELISA), radioimmunoassay, chemiluminescent immunoassay and SPR based immunosensors but are complicated, costly and time consuming. There is urgent need for the development of low cost, user-friendly and quick sensors for PSA. Recently, we have developed a simple and cost-effective biosensor for detection of PSA based on novel graphene quantum dots decorated with gold nanorods (GQDs-AuNRs) and modified with PSA antibody coated over screen-printed electrodes. The detection of PSA is demonstrated using three electrochemical techniques cyclic voltammetry (CV), differential pulse voltammetry (DPV) and electrochemical impedance spectroscopy (EIS). A typical response for the PSA is shown in the figure based on EIS technique. The modification of screen printed electrodes with novel hybrid of graphene quantum dots-gold nanorods and simultaneous detection using three different techniques makes the sensor sensitive, reproducible and reliable. PSA immunosensor shows 0.14 ng ml-1 limit of detection, which is capable of prediction of any disorder or chances of PSA cancer.
Wen Liu
Beijing University of Chemical Technology, China
Title: Surface chemistry and electrode design for high performance Li-S battery

Biography:
Wen Liu has completed his studies at Beijing University of Chemical Technology, China
Abstract:
Based on the reaction of 16Li+S8↔8Li2S, Li-S battery reaches a high theoretical energy density of 2600 Wh/kg, which is several times higher than that of traditional lithium ion batteries (LIBs). The low cost, high capacity as well as environment-benignity makes Li-S battery as a strong candidate for next generation energy storage. However, the development of Li-S battery is severely hindered by several problems, including the low conductivity of sulfur cathode, volume variation during charge/discharge and dissolution of lithium polysulfide (LiPS). These drawbacks cause low utilization of sulfur and poor cycling performance of batteries. To overcome these obstacles, researchers pay attention to regulating the construction of sulfur cathode, using mesoporous materials, core-shell types carbon and graphene oxide etc. The carbon based materials have significant benefit on the conductivity of the electrode and suppress the polysulfide dissolution to some extent. But the nonpolar carbon intrinsically has poor interaction with LiPS and lithium sulfide. Moreover, the lithium dendrite growth and electrode pulverization during cycling gives rise to lower columbic efficiency and safety risks. Hence, the polysulfide trapping chemistry, sulfur electrode design and Li mental electrode protection are the key factors contributing to the cycling performance and stability of Li-S battery. Herein, we propose polar materials including inorganic mental oxides, metal phosphides and organic functional groups, which further hybrid with nano carbons to construct bifunctional host for sulfur electrode. On one hand, the polar sites can strongly absorb LiPS, so that the dissolution of LiPS and shuttling effect can be reduced. On the other hand, polysulfide after absorption can quickly reacted with electrons and Li-ions, therefore improving the reaction kinetics and eliminating the bulky dead sulfur formation. Consequently, the Li-S batteries with the high performance sulfur electrodes can stably run for over 1000 cycles. The mechanism of sulfur trapping chemistry was also revealed by x-ray photoelectron spectroscopy (XPS) characterization and theoretical calculation. In terms of Li metal anode, the huge volume variation during cycling cause electrode pulverization, which is especially serious when paired with high mass loading sulfur cathode. We demonstrate that nanostructuring is one of the key points to realize stable lithium metal anode. Different kinds of scaffold have been constructed with Li mental to reduce the volume variation and suppress dendrite growth. The lithiophilic treatment of scaffold leads to lithium uniformly deposit and nucleate on electrode surface. The dendrite-free lithium deposition also achieved through manipulation of Li-ions transport number by modifying a separator with metal–organic framework materials (MOFs).
Bernhard Schuster
University of Natural Resources and Life Sciences, Austria
Title: S-layer protein lattice as a key component in biosensor development

Biography:
Bernhard Schuster holds an Associate Professor position for Molecular Nanotechnology and Biomimetic at the Department of Nanobiotechnology, University of Natural Resources and Life Sciences, Vienna, Austria. His main research interests focus on biomimetics, nanobiotechnology, cell envelope mimics and in particular functional supported lipid membranes and bio-inspired S-layer protein- and membrane protein-based sensors. His skills include recrystallization and modification of S-layer proteins, formation techniques for model lipid membranes, surface -sensitive and electrochemical techniques and the reconstitution and analysis of membrane-active peptides and membrane proteins. He is an Editorial Board Member of seven international peer-reviewed journals and filed two international patents. He published about 100 papers in peer-reviewed journals and book chapters and gave more than 120 (invited) contributions to international conferences. His total citations are 1.808; average citation per item: 27; h-index: 27. He serves as Ad-Hoc Reviewer of more than 20 papers per year.
Abstract:
Statement of the Problem: Combining biological with electronic components is a very challenging approach because it allows the design of ultra-small biosensors with unsurpassed specificity and sensitivity. However, many biomolecules lose their structure and/or function when randomly immobilized on inorganic surfaces. Hence, there is a strong need for robust self-assembling biomolecules, which attract great attention as surfaces and interfaces can be functionalized and patterned in a bottom-up approach.
Methodology: Crystalline cell surface layer (S-layer) proteins, which constitute the outermost cell envelope structure of bacteria and archaea, are very promising and versatile components in this respect for the fabrication of biosensors. S-layer proteins show the ability to self-assemble in-vitro on many surfaces and interfaces to form a crystalline two-dimensional protein lattice.
Findings: The S-layer lattice on the surface of a biosensor becomes part of the interface architecture linking the bioreceptor to the transducer interface, which may cause signal amplification. The S-layer lattice as ultrathin, highly porous structure with functional groups in a well-defined spatial distribution and orientation and an overall anti-fouling characteristics can significantly raise the limit in terms of variety and ease of bioreceptor immobilization, compactness and alignment of molecule arrangement, specificity, and sensitivity. Moreover, mimicking the supramolecular building principle of archaeal cell envelopes, comprising of a plasma membrane and an attached S-layer lattice allow the fabrication of S-layer supported lipid membranes. In the latter, membrane-active peptides and membrane proteins can be reconstituted and utilized as highly sensitive bioreceptors.
Conclusion & Significance: S-layer proteins bridge the biological with the inorganic world and hence, fulfill key requirements as immobilization matrices and patterning elements for the production of biosensors. This presentation summarizes examples for the successful implementation of bacterial S-layer protein lattices on biosensor surfaces in order to give an overview on the application potential of these bioinspired S-layer protein-based biosensors.
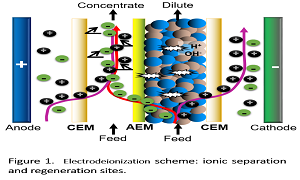
Lucia Alvarado
Guanajuato University, Mexico
Title: Electrodialysis and electrodeionization applied to remove Cr (III)

Biography:
Lucía Alvarado has her expertise in Electrochemical Separation Systems which as Electrodialysis and Electrodeionization and green chemistry. She had contribute in works testing and characterizing new carbon materials and nanomaterials. The main research is designing treatment systems to remove metallic ions from wastewater and getting pure water. Currently, her adscription is as full professor in Guanajuato University, Mex., Department of Mining, Metallurgy and Geology. She is active member in the International Society of Electrochemistry and has been reviewer for Elsevier: Electrochimica Acta and Separation and Purification Technology, also reviewer of some projects for the National Council of Science of Technology in Mexico
Abstract:
The development of new strategies to remove ionic metals from wastewater has been became in a global need. To design closed circuits that avoid release these compounds to the environment, from the environmental and production point of view, become each time more necessary. In this way, different kind of methods to remove metals are utilized, such as are the membranes systems. In this context, Electrodialysis and Electrodeionization are electrochemical process of membranes, which them have the capability to remove ionic species. This is why, both methods are available to be use as treatment of water polluted with heavy metals, without to generate wastes. In this way, the aim of the present work is to show the results of the use of these technologies applied to the remove of Trivalent Chromium from synthetic solutions, and evaluate their performance. Commercial membranes and resins were tested during the respective process in order to get advantages or disadvantages, when is used 100 or 1000 ppm Cr (III) solutions. The results presents some advantages for ED using bigger concentrations, meanwhile EDI is much better working low concentrations, spending less energy.
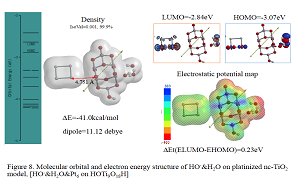
Shozo Yanagida
Osaka University, Japan
Title: Verification of photo-splitting of H2O to HOOH and H2 as initial photoproducts

Biography:
Shozo Yanagida is an Emeritus Professor of Osaka University and a Research Director of Research Association for Technological Innovation of Organic Photovoltaics (RATO) of University of Tokyo. Since he was promoted to a Professor of newly established Koza (research course) of Graduate School of Engineering in Osaka University (1980), he had contributed to photochemical conversion of solar energy, e.g., excellent photocatalysis of both nano-sized (quantized) ZnS and poly- & oligo-paraphenylene. When he was staying at SERI (now ENREL) as a Visiting Professor of Dr. A. Nozik’s group in 1984, he understood that organic molecules and their aggregates are kinds of quantum dots themselves. He has his expertise in evaluation of dye-sensitized solar cells, i.e., molecular structured photovoltaics, and enthusiasm in improving photo-conversion efficiency and long-term durability of solar cells on the basis of density-functional theory based molecular modeling.
Abstract:
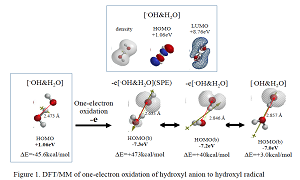
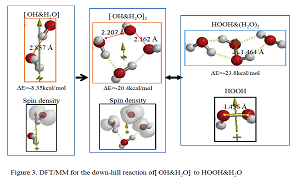
Most electrochemists and biochemists had a mindset that water oxidation yields oxygen molecules. However, Nosaka and his wife reports on generation and detection of reactive oxygen species such as HO. and HOOH in photocatalysis. We verified on the basis of density functional theory-based molecular modeling (DFT/MM) for photoelectrochemical H2O photo-splitting systems that formation of HOOH only under photo-irradiated and highly negative bias conditions. Further literature survey revealed that, in alkali aqueous solutions (pH 8~11.5), Pt-loaded nc-TiO2 catalyzes effective H2O photo splitting to HOOH and H2 as initial products. Figure 8 shows successful DFT/MM for an aggregate induced by van-der-Waals-Coulomb interactions (vdW&Clmb) between HOTi9O18H as a model of nc-TiO2 photocatalyst, HO-&H2O as an alkali water model, and Pt6 as platinum cluster model. Effective photoelectron transfer is verified from [HO-&H2O] to Pt6 for production of H2 on Pt and hydroxyl radical of [HO. & H2O] on nc-TiO2. Figure 1 shows DFT/MM for exothermic one-electron oxidation of alkali water model of hydrated hydroxide anion, [HO- & H2O] to hydroxyl radical of [HO. & H2O]. Figure 2 shows DFT/MM for exothermic vdW&Clmb- induced dimerization of the radical of [HO. & H2O], verifying that oxidation of [HO- & H2O] to HOOH & (H2O)2 via vdW&Clmb dimerization on nc-TiO2. Driving force of photo splitting will be verified as due to highly exothermic electron transfer reaction to Pt6 on nc-TiO2

Biography:
Ulrike Langklotz works in the field of Electrochemistry, mainly for applications in the field of energy storage, for ten years. The general attempt bases on the complementation of electrochemical results with suitable non-electrochemical measuring methods, e.g. spectroscopy. Main topics are the preparation and characterization of thin dielectric oxide films as well as the investigation of electrode materials used in lithium ion and lithium sulphur batteries. Recently, the investigation of nanostructured silicon as anode material was one main topic
Abstract:
Carbon coated silicon nanowires (SiNW) are the nanostructure of choice for binder-free high capacity anodes. These anodes with adjusted active mass loading and advanced morphology were grown by chemical vapor deposition (CVD). The high mass loadings (up to 6 mgSi/cm²) in combination with the lightweight carbon foil used as substrate and current collector predestines these anodes for applications where high energy densities are required, e.g. for automotive applications. The requirements in high power devices are especially challenging with respect to the (de)lithiation reactions. Thus, (complex) carbon coated SiNW anodes with varying morphology and mass loading are examined regarding their performance as well as the kinetics of the charge-discharge reactions. The specific capacity and cycle stability as well as the achievable C-rates strictly depend on these parameters. The anodes offer capacities up to 2 mAhcm-2, initial coulomb efficiencies higher than 80% and capacity fading of less than 10% over 100 cycles. The established process with high uniformity allows detailed examinations of the charge-discharge curves of samples with tuned properties and clearly shows an effect of the SiNW morphology on the phase transitions in the initial cycles, which in turn can be crucial regarding the degradation behavior of the anodes. Finally, galvanostatic intermittent titration technique (GITT) is applied to analyze the charge transfer and diffusion overpotentials of the (de)lithiation reaction. The overpotentials are basic kinetic parameters of these reactions, and they enable the estimation of the rate determining processes.
Thomas F Jaramillo
Stanford University, USA
Title: Design and development of catalyst materials for the production of fuels and chemicals in a sustainable manner

Biography:
Thomas F Jaramillo is an Associate Professor of Chemical Engineering at Stanford University and the Deputy Director of Experiments at the SUNCAT Center for Interface Science and Catalysis, a partnership between the School of Engineering at Stanford and the SLAC National Accelerator Laboratory. He earned his BS in Chemical Engineering at Stanford, followed by MS and PhD degrees in Chemical Engineering from the University of California at Santa Barbara. He then conducted research at the Technical University of Denmark as a Hans Christian Ørsted Postdoctoral Fellow prior to joining Stanford’s Faculty in 2007. His research efforts are aimed at developing materials and processes for sustainable chemical transformations related to energy conversion. He has earned a number of honors and awards for his efforts, including the Presidential Early Career Award for Scientists & Engineers (PECASE, 2011
Abstract:
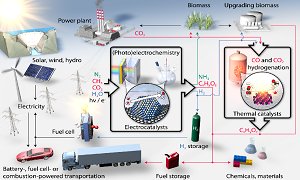
Statement of the Problem: The vast majority of fuels and chemicals that are produced and consumed across the globe today are derived from fossil fuels: oil, coal, and natural gas. The long list includes conventional liquid fuels such as gasoline, diesel, and jet fuel, in addition to many other products such as plastics (e.g. polyethylene) and fertilizer (i.e. ammonia, NH3). Society has benefitted tremendously from the science and engineering efforts that have brought these crucial products to market at a global scale, however continuing to use fossil-based resources at such high rates could potentially lead to troubling consequences ahead. This motivates the development of new chemical processes to produce the same kinds of fuels and chemicals that we rely on, using renewable energy and sustainable feedstocks instead.
Methodology & Theoretical Orientation: We seek to employ solar and wind energy to power the production of fuels and chemicals in a sustainable manner, largely motivated by the dropping costs of renewable electricity, the growing penetration of renewables into energy markets, and the need for storing variable electricity.
Findings: Catalyst materials have been developed capable of driving important chemical transformations in a sustainable manner involving electricity. Specific examples include the production of hydrogen (H2), carbon-based products (e.g. hydrocarbons, alcohols), ammonia (NH3) fertilizer, and hydrogen peroxide (H2O2).
Conclusion & Significance: The development of catalysts with appropriate properties can serve as the basis of new, renewable pathways to produce the large-scale fuels and chemicals that could play a major role in reaching sustainability goals for the globe.
Stefano Carli
Italian Institute of Technology, Italy
Title: New poly(3,4-ethylenedioxythiophene) coatings for neural recording and stimulation

Biography:
Stefano Carli has completed MSc and PhD in Chemistry from the University of Ferrara, Italy. He has devoted his PhD investigations to the development of new photochromic dyads. From 2009 to 2016 his research activity was oriented on dye sensitized solar cells, perovskite solar cells, as well as on new catalysts for light-induced water splitting. Currently he is a Postdoctoral Researcher at the Italian Institute of Technology and his research is focused on the development of new smart materials for neural sensing and stimulation. One of the main concerns of his research is the reduction of tissue inflammation as a consequence of neural probes implantation. For this purpose, new drug release systems from conductive polymers (PEDOT) are under investigation.
Abstract:
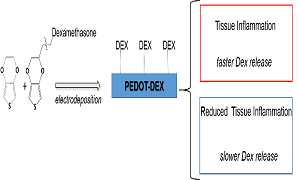
Statement of the Problem: The development of implantable neural microelectrodes has revolutionized the field of biomedical applications by enabling bidirectional communication with the nervous system at high resolution. Unfortunately, one of the main concerns related to chronically implanted neural microelectrodes is related to the adverse reaction of the surrounding tissue, which is known to encapsulate the neural microelectrodes after few weeks post implantation, leading to significant worsening of recording/stimulation quality. Among various approaches aimed to minimize inflammatory reaction and gliosis while preserving the electrochemical integrity of microelectrodes, the possibility of delivering anti-inflammatory drugs from the surface of neural implants represents a challenging strategy. For this purpose, the conductive polymer poly(3,4-ethylenedioxythiophene) (PEDOT), is commonly electrodeposited onto the microelectrodes in conjunction with the negatively charged dexamethasone sodium phosphate (Dex-P). Following this methodology, the drug release can be promoted by applying a cathodic trigger that reduces PEDOT to its neutral state, while enabling the free diffusion of the drug. Unfortunately, the inclusion of Dex-P as a dopant has been reported to negatively affect both electrochemical properties and stability of PEDOT coatings.
Methodology & Theoretical Orientation: In this study, for the first time, the anti-inflammatory drug dexamethasone (Dex) was chemically anchored to the surface of electrodeposited PEDOT, thereby enabling the drug release upon the hydrolysis of the chemical bond between Dex and the PEDOT film. This approach would account for a self-adjusting release system that promotes the delivery of the drug by local changes in the biologic environment.
Conclusion & Significance: The big challenge of this study was to realize self-adjusting release of drugs by neural implants, as a consequence of post implantation inflammatory biological triggers. Here we found that the covalent bond between Dex and PEDOT composite coatings can account for a biologically controlled drug release system.
Aurelien Habrioux
University of Poitiers, France
Title: Oxygen evolution reaction at the surface of nickel cobaltites: The impact of surface restructuring phenomena on the activity

Biography:
Aurélien Habrioux (Associate Professor) has an expertise in electrocatalysis and in materials science. His research interests deal with the design and development of novel non-noble electrocatalysts for reactions such as oxygen reduction reaction and oxygen evolution reaction in alkaline medium. He is especially interested in scrutinizing and explaining surface restructuring phenomena affecting catalysts surface and occurring upon working conditions. He has been coordinating researches aiming at developing transition metal oxides supported heteroatom doped graphene-based materials for the reversible air electrode of high energy density Li-air and Zn-air batteries. He has also been working on the understanding of the effect of the active phase/substrate interaction on the electrocatalytic activity.
Abstract:
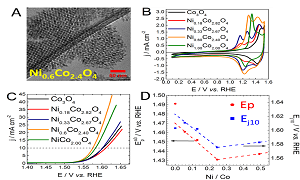
The storage of intermittent renewable energies requires the implementation of efficient energy storage systems. These systems must allow converting renewable energies into sustainable energetic vectors (hydrogen, electron). For this purpose, the oxygen evolution reaction (OER) plays an important role. OER possesses a sluggish kinetics that can be enhanced by using a catalyst exhibiting reliable surface composition and morphostructural properties. To limit the use of scarce noble metals, the synthesis of effective 3d transition metal oxide-based catalysts is of interest. As activity and stability of materials depend on their composition and morphostructural properties, the synthesis of well-defined catalysts is of utmost importance. To this end nanocasting approach constitutes an interesting pathway. In this study, NixCo3-xO4 materials have been synthesized by replicating ordered mesoporous silica templates. Materials were investigated using numerous physico-chemical techniques such as x-ray induced photoelectron spectroscopy (XPS), high resolution transmission electron microscopy, x-ray diffraction and Raman spectroscopy. Evidences from XPS and Raman measurements reveal that the different catalysts surfaces are hydroxylated. A particular attention was paid to restructuring phenomena occurring upon potential cycling and responsible for greatly improving the OER activity. These restructuring phenomena were evidenced using post-mortem Raman spectroscopy and XPS. It was observed that the intrinsic activity of the different restructured catalysts depends on the incorporated nickel amount and correlates with the CoIII/CoIV peak potential. The modulation of CoIII/CoIV peak potential is explained by changes in the chemical environment of surface Co atoms and results in the formation of nickel/cobalt oxy-hydroxide. Nickel indeed modulates the electronic properties of the Co active site and allows improving the OER activity of electrode materials. The catalysts described in this presentation are moreover very efficient since after surface restructuring, the over potential at 10 mA.cm-2 is as low as 310 mV.
M E Henry Bergmann
Anhalt University of Applied Sciences, Germany
Title: (Electro)chemical water disinfection – challenges for the 21st century

Biography:
M E Henry Bergmann has his 35-years expertise in electrochemical engineering that is recently focused on methods of drinking and process water disinfection. His technological approach for small and medium-size treatment devices respects the new European Biocide Regulation and the practical needs as well - against the background of avoiding or minimizing chlorination, and the formation of hazardous by-products. New (electro)chemical technologies are suggested basing on the use of chlorine dioxide, physically or electrochemically generated ozone, and combined methods.
Abstract:
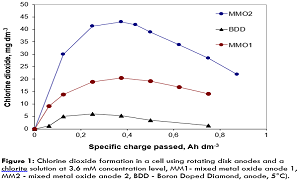
Whereas worldwide hundreds of millions of people do not have access to safe drinking water, in developed countries current research and practice are oriented towards problems of micropolutants and disinfection by-products formed at µmole-per litre level. Another special subject of activities is the low-chemical disinfection of process waters in recirculation and cooling systems. Although direct electrochemical drinking water disinfection is applied for decades of years, conditions for avoiding over chlorination and disinfection by-products could only be clarified in the recent years. A new approach is the electrochemical generation of chlorine dioxide (ClO2) at mm concentration level. It is well known that ClO2 has much lower organic by-product formation potential compared to free active chlorine. The challenge is to minimize chlorite, chlorate and perchlorate in the solutions obtained. The high chlorite reactivity often causes maxima in ClO2 formation. Highly active anodes dramatically reduce the generation of chlorine dioxide due to parasitic reactions. Solutions for inorganic electrolysis and disinfection by-products can be found by analysing and studying influence parameters such as temperature, electrode material, counter electrode material and cell construction. ClO2--to-ClO2 yields of 75% are possible at the moment. Furthermore, it is discussed if combined methods may contribute to lower by-product formation. A simple variant is the combined chlorine-chlorine dioxide formation. The combination of ozone and chlorine dioxide in situ is another interesting option. First own experiments have shown that all initial chlorite can be converted to chlorine dioxide. Lower temperatures in the range of 5℃ are preferred reaction conditions. It can be stated that in the future improved regulations and inline analysis methods have to be applied for safer disinfection. Means of digitalization could support the process.
E Fillis Tsirakis
Max Planck Institute for Solid State Research, Germany
Title: Integration of functional liquids in solid-state electronic circuits

Biography:
E Fillis Tsirakis completed his studies in Max Planck Institute for Solid State Research, Germany.
Abstract:
Field-effect gating with solid dielectrics is the basis of modern electronics. It is a technique that is most successfully used in integrated circuits. Here, we present our work on realizing solid-state heterostructures with fully integrated liquids, opening a brand new phase-space of materials for integrated circuits. Gating with liquid electrolytes in field-effect transistors offers clean contact-electrolyte interfaces and higher polarizations than in conventional, all-solid-state architectures. We demonstrate the fabrication of electronic devices such as capacitors and field-effect transistors with integrated, patterned aqueous-NaCl solutions, which are of equal quality or even outperform standard, bulk electrolyte devices. Our work opens a new route to the exploitation of solid–liquid interfaces in integrated functional devices.
Vladimir S Bystrov
Keldysh Institute of Applied Mathematics RAS, Russia
Title: Computational studies of ferroelectric composites and thin films containing polyvinylidene fluoride (PVDF) and graphene/graphene oxide

Biography:
Vladimir S Bystrov has completed PhD, Dr. Habil.Phys. Dr.Sci. Phys. & Math. from Russian Academy of Sciences. Since 1993, he has his expertise in various fields of computational molecular modeling, computational exploration and computer simulation of nonlinear multifunctional nanomaterials and different organic & bio-molecular nano-structures such as: bioferroelectric & polymer PVDF/PVDF-TrFE thin ferroelectric films, graphene/oxide graphene and related polar composite nanomaterials; amino acids (glycine, etc.), peptides nanotubes, thymine & DNA; hydroxyapatite (HAP) & nanoparticles, etc. Computational studies of nanostructures were made using the molecular mechanics, quantum-chemical calculations (ab initio, DFT, semi-empirical methods), molecular dynamics (MD) on the base of various software (HyperChem, AIMPRO, VASP, etc.) and clusters in Russia IMPB & KIAM, Linux cluster in University of Aveiro, Portugal. He is a Head of the Group for Computer Modelling of Nanostructures and Biosystems of IMPB-KIAM RAS, Pushchino.
Abstract:
Computational molecular investigations and experimental studies of the ferroelectric properties of new composite nanomaterials based on polymer ferroelectrics and graphene/graphene oxide are presented. Main results of the computational molecular modeling of various nanostructures and the piezoelectric properties of the composites from polyvinylidene fluoride (PVDF)/poly(vinylidene fluoride-trifluoroethylene) (P(VDF-TrFE)) films and graphene/graphene oxide (G/GO) were reviewed and analyzed in comparison with the experimental data at the nanoscale, particularly with atomic force and piezo-response force microscopy (AFM/PFM) data. The performed computational molecular modeling of the graphene/graphene oxide (G/GO) and PVDF ferroelectric polymer composite nanostructures were studied by the different methods using HyperChem tool: molecular mechanics (MM) methods (BIO CHARM), quantum mechanical (QM) calculations based on density functional theory and semi-empirical PM3 method. Experimentally the switching behavior, piezoelectric response, dielectric permittivity and mechanical properties of the films were investigated and found to depend on the presence of G/GO concentration variation. Experimental results qualitatively correlate with those obtained in the calculations. Particularly, computed data of the piezoelectric coefficients d33 for developed PVDF-G/GO models are in line with observed experimental behavior with concentration changes of GO components. Further development with several multilayered GO nanostructures and inserted PVDF chain and layers, having new curved structures after optimization are considered and discussed. The properties of these investigated nanostructures with the GO content dependence for these composites are analyzed. The results obtained in the reviewed and analyzed present study provide important insights into our understanding of the mechanisms of piezoelectricity in such new nanocomposites give us new prospective for further creation, development and applications of novel ferroelectric polymer–graphene/graphene oxide nanocomposites as multifunctional nanomaterials.
M Lapkowski
Silesian University of Technology, Poland
Title: Electrochemistry of bipolar s-tetrazine derivatives

Biography:
M Lapkowski is a Full Professor of Silesian University of Technology and Director of Department of Physical Chemistry of Polymers, Faculty of Chemistry, Polish Academy of Science. His fields of research activity are the synthesis of electronic conducting polymers, the physicochemical characterization of polymers, oligomers and composite materials, synthesis and characterization of new organic materials for optoelectronics and photovoltaic. He was an Assistant Professor in l'Université de Nantes, (France), and Invited Professor in l’Ecole Normale Superieur de Cachan, (France), University of Sao Paolo, Brazil, University of Woolongong, Australia and Tohoku University, Japan. He is a Member of International Society of Electrochemistry since 2005.
Abstract:
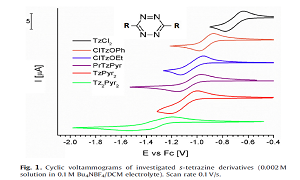
1,2,4,5-tetrazine, especially 3,6-disubstituted derivatives are very well known materials, in particular, in the field of the energetic chemistry. However, s-tetrazine ring has a number of other interesting properties. It is electroactive, with a very high electron affinity, furthermore it is highly colored. It is the smallest fluorophore, which makes s-tetrazine derivatives very promising molecules for active layers in optoelectronics devices such as organic light emitted diodes (OLED), electrofluorochromic and electrochromic windows. The functionalization of the ring with electron-donating group leads to obtain the donor-acceptor-donor (D-A-D) type of structure, which can serve as both: electron as well as hole transporting materials. Electrochemistry is a suitable method for characterization of new electroactive organic materials for optoelectronic and electronic applications. It provides lots of information about redox properties, stability, the conversion and storage of energy, etc. It also allows to determine the electron affinity and ionization energy of investigated compounds, parameters which are correlated with energies of HOMO and LUMO level, which need to be determined if materials are investigated towards optoelectronic applications. In this work we present the electrochemical and spectroelectrochemical characterization of bipolar s-tetrazine derivatives. The characterizations of studied compounds were performed using: electrochemical techniques including cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements; spectroelectrochemical investigations such as UV-Vis, EPR, Raman and fluorescence spectroelectrochemistry. The electrochemical characterization indicated that a few of studied s-tetrazine derivatives undergo electrochemical polymerization (oligomerization) which is rarely observed in this group of compounds. Hence, monomers as well as electrochemical obtained polymers were studied. Their redox processes have been investigated by in situ UV-Vis and EPR spectroscopy. A huge effect of chemical structure on the electrochemical properties was observed. Introduction of oxygen atom as a linker between donor and acceptor part of molecules resulted in obtained thin polymer layer with unique properties.
Guowei Li
Max Planck Institute for Chemical Physics of Solids, Germany
Title: Interfacially engineering topological semimetal MoP into a superior electrocatalyst for hydrogen evolution

Biography:
Guowei Li received his Master’s degree in Materials Science from Jiangsu University in 2011 with Prof. Changsheng Li. He then moved to the University of Groningen, the Netherlands, and was awarded the PhD degree under the supervision of Dr. Graeme R Blake and Prof. Thomas T M Palstra in 2016. Since then, he joined the Max Planck Institute for Chemical Physics of Solids, Dresden as a Postdoctoral Fellow in the group of Prof. Claudia Felser. His research interests focus on the magnetic and electrical transport properties of chalcogenides and topological materials, from synthesis to applications in clean energy harvesting and conversion.
Abstract:
Materials in topological states are endowed with many exotic properties such as extremely large magnetoresistance, high conductivity, and intrinsic Hall effects. However, the effect of such appealing properties on electrocatalysis still remains elusive. Recently, the observation of exceedingly high conductivity and Weyl nodes in MoP provide us with a modeling catalyst for revealing the correlation between topological states and electrocatalytic activity and designing high-performance electrocatalyst for hydrogen evolution reaction (HER). MoP encapsulated in Mo, P co-doped carbon layer (MoP@C) was thus synthesized and exhibits outstanding electrocatalytic HER performance with an extremely low overpotential of only 49 mV at a current density of 10 mA/cm2 and a decreased Tafel slope of 54 mV/dec. in alkaline medium. Such superior HER activity of the MoP@C exceeds those of the-state-of-art non-noble metal-based HER electrocatalysts and even comparable to that of the Pt/C catalyst. As a topological semimetal, MoP manifests a very high conductivity (8.2 μΩ at 300 K) and mobility (up to 10 cm2/VS at 300 K) due to the topologically protected triple point fermions and complex Fermi surface. Meanwhile, the rich P-C and Mo-C bonds in the interfaces between the carbon layer and MoP modulates the band structure of MoP@C and eventually facilitates the fast electron transfer, accumulation, and subsequent de-localization, which are responsible for the excellent HER activity.
- Environmental Electrochemistry | Electronic Materials and Processing | Electrochemical Water Treatment | Dielectric Science and Materials | Electrochemical Engineering | Physical and Analytical Electrochemistry
Location: Olimpica 1

Chair
Elod L Gyenge
University of British Columbia, Canada

Co-Chair
Peggy Gunkel Grillon
University of New-Caledonia, New Caledonia
Session Introduction
Elod L Gyenge
University of British Columbia, Canada
Title: Novel manganese dioxide-based electrocatalyst formulations for bifunctional oxygen reduction and evolution reaction activity

Biography:
Elod L Gyenge is a Professor in the Department of Chemical and Biological Engineering and Clean Energy Research Centre at the University of British Columbia, Vancouver, Canada. His research is focused on electrocatalysis and electrochemical engineering for improving the performance of electrochemical power sources and electrosynthetic processes. His research led to many innovations for a variety of electrochemical systems including diverse fuel cells and rechargeable batteries, and electrosynthesis of hydrogen peroxide. The research materialized in over 75 refereed research publications in peer reviewed journals, over 40 invited presentations and 10 patents and patent applications. He has received a number of awards and recognitions, among them the Japanese Society for Promotion of Science (JSPS) Fellowship at Osaka University and Elisabeth and Leslie Gould Endowed Professorship at UBC (2007-2014). Since 2016 he is also cross-appointed Professor in the Graduate School of Engineering at Osaka University, Japan. He is a Co-Founder of two companies: Catalyst Square Materials Ltd. and Agora Energy Technologies Ltd.
Abstract:
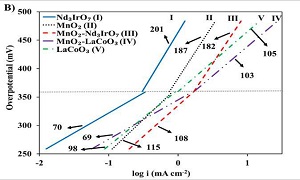
Development of highly active, durable and cost-efficient bifunctional electrocatalysts for the oxygen reduction and evolution reactions (ORR and OER) is of outmost importance for commercialization of rechargeable metal-air batteries (e.g., Zn-air, Al-air, Mg-air, Li-air) and regenerative H2-O2 fuel cells. Manganese dioxide (MnO2), a low cost and abundant material, has been intensely studied as ORR electrocatalyst in alkaline media. Regarding the bifunctional ORR and OER electrocatalytic performance, however, enhancement of the activity (e.g., lower surface overpotential at practical current densities above 100 mA cm-2) and improvement of the long-term stability are required for potential implementation in commercial systems. The purpose of this study is to present novel approaches for tuning the MnO2 performance with co-catalyst addition, potassium ion doping and support effect (e.g., graphene and graphitized carbon). The combination of MnO2 with structurally different oxide co-catalysts such as perovskite (LaCoO3) or fluorite-type oxide (Nd3IrO7) produces a synergistic catalytic effect improving the bifunctional (ORR and OER) activity compared to the individual oxides. Doping of the oxide catalyst with potassium ions, either by long-term exposure to 6 M KOH or potential driven insertion (PDI), increases further the activity and durability as revealed in accelerated degradation experiments. Optimizing the MnO2 electrodeposition conditions can produce nanostructured morphologies that are favorable for ORR and OER activity. The electrochemical studies are supported by extensive surface analysis (SEM, TEM, XPS, EDX, EELS). This work reveals new oxygen electrode catalyst formulations for rechargeable metal-air batteries and regenerative fuel cells.
Peggy Gunkel Grillon
University of New-Caledonia, New Caledonia
Title: Calcareous electrochemical precipitation, a new method to trap dissolved metallic contaminants in seawater

Biography:
Peggy Gunkel Grillon is an Environmental Chemist having expertise in heavy metals and their contamination, bioavailability and mobility in the environment. She is an Assistant Professor since 2008 and Deputy Director of ISEA laboratory (Institut des Sciences Exactes et Appliquées) at the University of New Caledonia. She has a keen interest in understanding heavy metals behavior in the environment for a modern society concerned with sustainable development. Recently, her activities also include the development of remediation techniques. With her colleagues she’s been working for 4 years on the electrochemical precipitation of heavy metals in seawater to trap dissolved metallic contaminants.
Abstract:
The contamination of coastal waters by trace metals is an important worldwide concern since they may significantly affect marine ecosystems. A novel use of the calcareous deposit formed on a metallic structure is proposed to trap metallic contaminants in seawater. It is the same deposit that builds up in many tea kettles or water pipes in areas where calcium-rich water is the norm. Whereas the calcareous deposition is a common problem for many people, we transformed this problem into a solution to trap metals. The calcareous deposit is formed in seawater by imposing a current on a galvanized steel electrode. The working electrode’s potential reaches potential in the water reduction range. This reaction causes pH increase at the seawater/metal interface, inducing calcium and magnesium precipitation. A voluminous calcareous deposit composed of CaCO3 and Mg(OH)2 grows with polarization time. Experiments conducted in situ revealed that many metals can also be trapped. In order to better control and understand the mechanisms, lab-experiments were performed in artificial seawater. We first decided to study nickel trapping since nickel mining activities in New Caledonia are causing the subsequent pollution of local coastal waters. Artificial seawater was doped with NiCl2(s) and analysis revealed that Ni is trapped mainly as β-Ni(OH)2. Ni content increases with the initial Ni concentration in the electrolyte. Up to 24% in weight of Ni is trapped in the deposit after seven days of polarization. The calcareous deposit appears like a simple implementation with just a metallic structure immerged in seawater and connected to an electrical circuit which can be charged by renewable energy. This electrochemical method is thus a promising and cheap clean-up device for remediation of contaminated seawater.

Peggy Gunkel Grillon
University of New-Caledonia, New Caledonia
Title: Calcareous electrochemical precipitation, a new method to trap dissolved metallic contaminants in seawater

Biography:
Peggy Gunkel Grillon is an Environmental Chemist having expertise in heavy metals and their contamination, bioavailability and mobility in the environment. She is an Assistant Professor since 2008 and Deputy Director of ISEA laboratory (Institut des Sciences Exactes et Appliquées) at the University of New Caledonia. She has a keen interest in understanding heavy metals behavior in the environment for a modern society concerned with sustainable development. Recently, her activities also include the development of remediation techniques. With her colleagues she’s been working for 4 years on the electrochemical precipitation of heavy metals in seawater to trap dissolved metallic contaminants.
Abstract:


Biography:
Roman Korobko received his BA in Chemistry and BSc in Materials Engineering in Technion – Israel Institute of Technology in 2003. He continued his studies in the Faculty of Chemistry of the Weizmann Institute of Science. He earned an MSc in the theme of Molecular Electronics in 2009 and PhD on controlling the elastic properties of ceramics with an external electric field in 2014. Until 2017 he was conducting a Postdoctoral Research in ETH Zurich in the field of Memristive Materials. Now he holds the position of Senior Intern in Inharmonicity of Functional Materials group at Weizmann Institute of Science. His research interests include the elastic, electronic, electromechanical and memristive properties of dielectrics, focusing on solid oxide ionic conductors. He was a recipient of the 2012 Acta student award and E-MRS 2013 graduate student award.
Abstract:
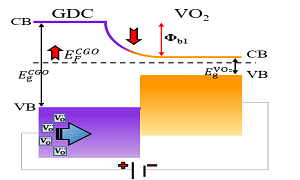
Electrochemical resistive switches operating on ionic carriers, sometimes named memristors, may revolutionize the future electronics as the next generation building blocks of non-volatile memory and neuromorphic computing replacing electronically operated classic transistor structures. Despite an extensive research performed on solid oxide materials, the technology is still immature. Therefore, the exploration in the direction of understanding the mechanisms and adaptation of novel materials systems is ongoing. In this presentation, we show a study of memristive properties of Ce0.8Gd0.2O1.9/VO2 thin film system (Gd-doped ceria (GDC), and V4+ vanadia). Ceria is a well-studied ionic conductor that tolerates high percentage of mobile oxygen vacancies. Vanadia, as VO2 is famous for its metal-insulator transition, an ability to switch the resistance by several orders of magnitude by change of temperature, electromagnetic fields or mechanical strain beyond a sufficient transition level. Furthermore, ceria is a wide bandgap (~3 eV) and vanadia is a narrow bandgap n-type semiconductor (0.7 eV). Combination of these materials in one device seems incompatible for the conventional electronic materials strategy due to the dissimilar electric/dielectric properties. We show that integrating both oxides in the double layer device yields to synergetic memristive results, which are uncharacteristic neither for GDC nor for VO2 as oxide constituents. It was experimentally found that the conduction and the resistive switching are governed by the mass transport kinetics, which is a function of the applied voltage, the electric field and the voltage application rate. We suppose that the field-induced transport of oxygen vacancies to and from the ceria-vanadia interface modifies the electrically variable energy barrier, which tunability is responsible for the enhanced memristance effect.
Yan sun
Max Planck Institute for Chemical Physics of Solids, Germany
Title: Linear response in topological semimetals

Biography:
Yan Sun has his research interests mainly focus on the theatrical study of topological materials. Through the analysis of the relationship between Berry curvature and band structure, we revealed strong spin Hall effect (SHE) and anomalous Hall effect (AHE) in WSMs and nodal line semimetals. The generalized relation between SHE/AHE and topological band structure suggests a way of the application of topological semimetals in spintronics. Fundamentally, the intrinsic AHE just depends on the symmetry of Berry curvature, but not the magnitude of net magnetic moments. Guiding by this principle, we have deeply studied two ideal strong AHE systems with vanishing net magnetic moment, non-collinear antiferromagnets (Mn3Ge) and compensated ferrimagnetic WSM (Ti2MnAl).
Abstract:
Topological insulators have been expected to be ideal spintronic materials due to the spin currents carried by the surface states with spin-momentum locking. However, the bulk doping problem still remains to be an obstacle that hinders such application. While this kind of problem is naturally avoided in topological semimetals due to the large anomalous Hall and spin Hall effect originated from the intrinsic bulk band structures. We have found that the strong spin Hall effect in TaAs is mainly dominated from the Weyl points and nodal-line-like Fermi surface, which implying a strong interplay between the topological band structure and Berry curvature in topological semimetals. With this guiding principle, we have successfully understood the strong spin Hall effect in IrO2 and found the nodal line band structures in it. Generalizing this principle to time reversal symmetry breaking system, we have predicted strong anomalous Hall effect in magnetic Weyl semimetal Co3Sn2S2, which was verified by our experimental collaborators. Owing to the low charge carrier density and large Berry curvature from the nodal line band structure, the anomalous Hall conductivity and anomalous Hall angle experimentally reach up to 1130 S/cm and 20% respectively. Further, the anomalous Hall effect can even exist with zero net moments in the absence of the symmetry operation that changes the sign of Berry curvature. And the anomalous Hall effect can be strongly enhanced by the special band structures of Weyl points and nodal lines. Following this guiding direction, we have predicted a strong anomalous Hall effect in the compensated ferrimagnetic Weyl semimetal Ti2MnAl with vanishing magnetic net moments. Our work is helpful for the comprehensive understanding of the linear response effect in topological materials and their future applications.
Mackenzie Honikel
Arizona State University, USA
Title: Electrochemical breast cancer screening technology facilitating earlier clinical diagnosis

Biography:
Mackenzie Honikel is a current PhD student in the School of Biological and Health Systems Engineering at Arizona State University, mentored by Dr. Jeffrey LaBelle. She graduated from Binghamton University in May 2016 with a Bachelor’s degree in Biomedical Engineering, with a concentration in biomedical devices. Her research background is in point-of-care diagnostics and she aims to continue this work during her doctoral training. Her current research focuses on the development of a continuous, implantable sensor platform for continuous monitoring throughout the episode of care for breast cancer patients.
Abstract:
Statement of the Problem: Breast cancer remains the second leading cause of cancer related death among women and accounts for nearly one in three cancer diagnoses. Advances in mammography have helped improve the early detection rate; however, noninvasive imaging modalities are unable to accurately identify the molecular subtype of the disease, therefore delaying treatment until further validation. In addition, technological advancements have increased annual screening costs, segregating lower income populations from proper preventative care. To facilitate earlier diagnosis and treatment, a point-of-care (POC) electrochemical biosensor is currently being pursued to provide immediate, sensitive and specific diagnostic information.
Methodology & Theoretical Orientation: Using electrochemical impedance spectroscopy (EIS) and a novel imaginary impedance algorithm a panel of biomarkers can be detected, simultaneously. Through the identification of a biomarker’s respective optimal binding frequency rapid signal acquisition is achievable, permitting signal deconvolution and robust characteristics.
Findings: Currently we have validated detection of a previously FDA approved biomarker on a benchtop electrode platform revealing low limits of quantification. Upon the characterization of other breast cancer indicative biomarkers, a multiplexed POC sensor will be developed and validated in complex media and clinical samples. Additionally, a screen-printed electrode platform and novel immobilization protocol will be adopted for increased feasibility in clinical use.
Conclusion & Significance: We propose that the developed technology in conjunction with electrochemical detection methodologies has profound applications in other medical conditions where a rapid diagnostic test could be useful in supplementing clinical diagnosis.
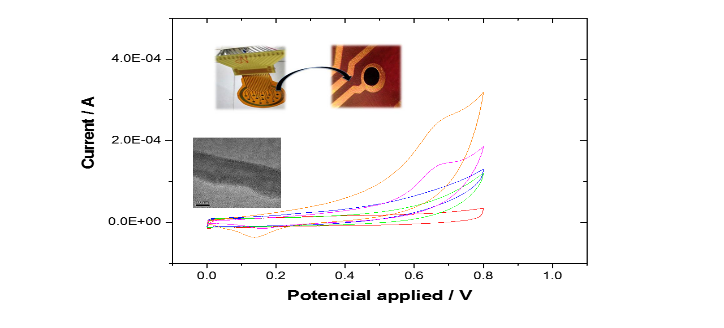
Atiweena Krittayavathananon
University of Oxford, UK
Title: Improving single carbon nanotube electrode contacts using molecular electronics

Biography:
Atiweena Krittayavathananon PhD has her expertise in Applied Electrochemistry, Electro-Analysis and Functional Materials. After years of experience in the research, she found new pathways for observing aggregation of 2D materials in colloids and suspensions using an electrochemical “nano-impacts” based on bridging impacts. The “nano-impacts” has proved to be an effective approach for investigating single nanoparticle behavior in solution phase. By using this technique, she creates a simple idea for minimizing contact resistance between catalysts and supporting electrode in the solution phase as presented in her talk.
Abstract:
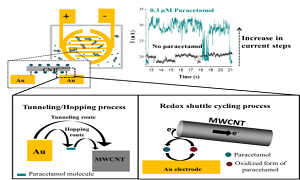
Carbon nanotubes (CNTs) and their derivatives are commonly applied as both catalyst supports and catalysts in many electronic devices. To achieve high-performance electronics, researchers have focused intensive efforts into developing the chemical and physical properties of new materials but largely ignore the potentially fundamental problem of forming a high-quality contact with the electrochemical substrate. When two materials are brought into contact, the junction causes a potential drop in the system resulting from a contact resistance. To understand the junction properties of metal/CNT interfaces, the nano-impact methodology has been developed as a route to measuring the resistance across individual CNT−electrode contacts. In these experiments, some of the CNTs in the solution phase form a bridge across two adjacent gold electrode contacts. An average bridging resistance for individual CNTs contact is 1.1±0.1×108 Ω. To improve the CNT-Au contact, we report the use of an electroactive species, acetaminophen, to modify the electrical connection between a carbon nanotube (CNT) and an electrode. By measuring the current signal across the bridge of single acetaminophen-modified CNT contact between the two microbands of the IDE-Au, the current response of acetaminophen modified on CNT is significant higher than the bare CNT, indicating that the electronic properties of the single CNT-Au contact are improved by modifying CNT with acetaminophen. It investigates that the adsorbed acetaminophen molecules contribute to promoting the electron transfer processes between the junctions of two materials.
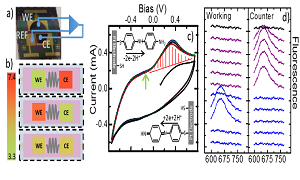
Vinita
Banaras Hindu University, India
Title: Nanocrystalline scaffold of AMT-Ag for electro-sensing of ciprofloxacin drug in biological fluid and pharmaceutical formulation

Biography:
Vinita has her expertise in synthesis of nanosized metal coordination polymers and metal nanoparticles for sensing application. Her system based on metal organic framework. She has built this system after 2 years of experience in research institutions. Her established system has potential for fabrication of high performance electrochemical sensors, biosensors and colorimetric sensors for the detection of biologically important drug and biomolecules. Her successful effort in the area of silver and palladium based coordination polymers synthesis and their sensing application has been recently recognized.
Abstract:
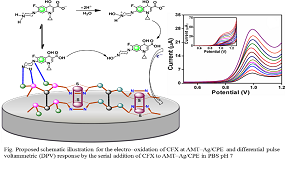
The advancement in the chemistry of the coordination polymers having designable architectures fabricated from functionalized building blocks is an emerging area from last two decades. The further challenges are the construction of coordination network assembly having electro-active nano-pores. We are first time exploring a nanocrystalline coordination polymer (NCCP) framework resulting from 2–amino–5–mercapto–1,3,4–thiadiazole (AMT) and silver nitrate. In the infinite polymer arrangement of AMT–Ag, silver (I) centers are bridged by tecton AMT through the amino linkage and exocyclic thiol. The grasped nano–sized granules of AMT–Ag are investigated by FE-SEM. The crystalline nature along with the oxidation state of silver is studied through XRD, TEM and XPS respectively. Additionally, the thermal stability and activation energy for thermal decomposition of NCCP are scrutinized by thermo–gravimetric analysis. Furthermore, the efficient electron transfer kinetics is probed by using Fe (II)/Fe (III) redox couple in phosphate buffer pH 7 via cyclic voltammetry. The excellent electroactivity is employed in the electro-detection of a biologically active drug molecule ciprofloxacin hydrochloride (CFX). The anodic peak current revealed a linear dependence with CFX concentration with sensitivity and limit of detection as 0.001 μA/μM and 5.0 nM, respectively. The effective assay of the drug is caused by the excellent electron channeling through the pores of polymeric nano–crystallites. Further, the concept is extended and established in the voltammetric detection of CFX in biological fluid and pharmaceutical formulation by a considerably high sensitivity (0.002 mA/mM and 0.007 mA/mM) and the detection limit (22 nM and 60 nM) respectively. Our established system has potential for fabrication of high performance electro-chemical sensors for assay of biologically significant drug molecule.
Preeti Tiwari
Banaras Hindu University, India
Title: Ch@AgNPs modified SPGE: Voltammetric estimation of anti -HIV drug

Biography:
Preeti Tiwari has completed MSc in Chemistry and has her expertise in electro-chemical sensing, conducting polymers and nanomaterials. She is developing sensors for drugs (especially anti-cancerous and anti-HIV drugs). She is also working for the development of the handheld devices for drug detection.
Abstract:
Azidothymidine (AZT) is an anti-HIV drug used against the treatment of HIV-1 (human immunodeficiency virus-1) infections. HIV-1 is the major cause of AIDS in humans. This drug is used for the treatment of this immunosuppressive disease since 1987 and still it is one of the drugs of choice either given alone or in combination of some other drugs. This drug has a major disadvantage that its concentration more than 10 µM in human serum causes several side effects. So, its concentration has to be maintained in human serum at very low level. Various methods are evolved for the detection of this drug using various techniques like HPLC, HPTLC etc. Electro-chemical detection of this drug is highly advantageous as it takes less time and quick response. Various modifications are utilized for electro-chemical detection of AZT. We first time developed handheld device for the detection of this drug using modified screen printed graphite electrode (SPGE). For modification we synthesized chitosan capped silver nanoparticles (Ch@AgNPs) using one pot, facile chemical reduction method. This material is utilized for the fabrication of screen printed graphite electrode (SPGE) and modified SPGE nanostructures platform is further used for estimation of AZT using simple cyclic voltammetric techniques in phosphate buffer solution at pH 7.6. This method is the most advanced as it is helpful for the development of portable sensing probes.
Richa Mishra
Banaras Hindu University, India
Title: Surface induced assembly of PTZ-TCNQ complex and its charge transport characteristics

Biography:
Richa Mishra has her expertise in self-assembly and processing of various organic materials for large area film formation via various techniques such as spin coating, Langmuir-Blodgett and Langmuir-Schaeffer. Her successful effort in the area of ordered film fabrication of non-alkyl chain conducting polymers and other п- conjugated materials has been recently recognized.
Abstract:
Tailoring of physical properties of π-framework containing organic molecules by tuning their non-covalent interactions is an intensive research area. Non-covalent interactions among organic molecules can be intervened to assemble them preferably via solution based processing methods. These solution processable materials can then be assembled with varying morphology thus procuring appropriate physical properties requisite for the application desired. We are more interested in processing organic synthetic metals for large area thin film based device applications. Organic charge transfer materials particularly those containing 7,7,8,8-tetracyano-p-quinodimethane (TCNQ) as one precursor are in current scientific interest due to its intrinsic (TCNQÖ—¯) radical anion formation and astonishing electrical properties when complexed with strong donors like tetrathiafulvalene (TTF), phenothiazine (PTZ), etc. Besides these gains, these complexes carry a limitation of not forming flexible, uniform and ordered large area film in their pristine state thus restricting themselves in electronic device applications. Here, it is a motivation to investigate morphology controlled assembly of donor-acceptor PTZ-TCNQ charge transport complex on solid (hard) and liquid (soft) surfaces and their film formation. We have studied their surface (liquid and solid) induced assembly via spin casting and Langmuir technique. Films fabricated from both soft and hard surfaces depicted variation in crystalline morphology and surface topography that has been studied via SEM, TEM and AFM characterizations. These assembled large area films have also shown variation in electrochemical properties and charge transport characteristics as per their molecular arrangement and their stacking/orientation on the substrate. The main purpose of this study was to substantiate the significant effect of surface induced processing methodology on the assembly of the PTZ-TCNQ complex and its charge transport characteristics. We believe this study may open new dimension towards large area based organic electronics device application of TCNQ based organic metals.
Alay Patel
Pandit Deendayal Petroleum University, India
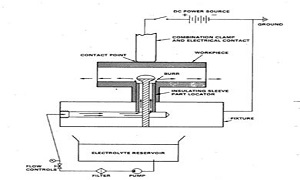
Title: Experimental study on conductivity versus concentration of electrolytes for electrochemical deburring process

Biography:
Alay Patel is pursuing his graduation in Mechanical Engineering at Pandit Deendayal Petroleum University, Gandhinagar, Gujarat, India. He has been appointed as an Undergraduate Research Assistant to a PhD scholar and has been working on the topic of Electrochemical Deburring for the past two and half years. Based on his passion and the opportunities provided at the institution, he has gained a good amount of knowledge base and research experience in manufacturing division of mechanical engineering. Other than that, he had participated in an industrial innovation competition organized by Larsen & Toubro (L&T) Technological Services, in which he secured a position in top 20 participants out of 7000, at the national level.
Abstract:
Statement of the Problem: Electrochemical deburring (ECD) is a widely popular process among industries to manufacture miniature parts and intricate components. Hence, it is important to optimize its process parameters to obtain high material removal rates and cost efficiencies. The domain of this paper focuses on the electrolyte types and control over the variation of its concentration during ECD operations. Here, a technique is developed to maintain a set value of electrolyte concentration based on its relation with the electrical conductivity of electrolytes.
Methodology & Theoretical Orientation: Sample testing solutions were prepared in laboratory for the electrolytes, sodium chloride and sodium nitrate. Conductivity and total dissolved solids (TDS) measurements were taken for each sample and recorded. Standard conductivity and TDS versus concentration charts were prepared corresponding to the measurements. Then the charts are trend-fitted to obtain certain empirical relations for the concerned parameters. These relations are then used to identify the value of conductivity by substituting the desired amount of concentration.
Conclusion & Significance: The measured values of conductivity and TDS for various concentrations of sodium chloride and sodium nitrate show a proportionate growth with respect to the concentrations. The interpolation models obtained from the plots can be utilized in industrial ECD operations to control and manipulate concentration of electrolytes. As it is a difficult task to maintain a set value of concentration in such applications, this technique can simplify it by monitoring the corresponding value of conductivity for the required concentration.
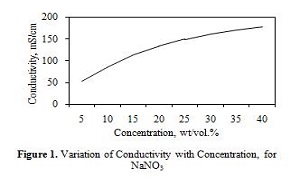
Dina U Alshimbayeva
Kazakh National Research Technical University, Kazakhstan
Title: Organization of chemical reactions by using the discrete properties of electric current

Biography:
Dina U Alshimbayeva is pursuing her PhD with specialization in Project Management. Her major is Oil and Gas Business. She is the Executor of research projects in alternative energy sources, geology and oil and gas industry, metallurgy fields. She has experience of commercialization of results of scientific research and technologies.
Abstract:
We investigated the mechanism of transport of electric current through the liquid. Early, Michael Faraday has concluded the discrete nature of electric current. Taking into account, the works of M Faraday a study on relationship between electric current and phase transitions was performed. It is shown that phase transitions are caused by electric current and they cause a change of electrical conductivity due to the orientation of elements of the microstructure and generate electron flow. The discrete character allows using pulse electric current for the organization of unusual reactions and technological processes. Transport of electric current through liquid depends also on a cell design for measurement of conductivity. Electric current can cause crystallization of components of liquid on vessel walls. Electrophysical characteristics of the components of liquid influence the electrochemical technologies. Researches of power manifestations during physical and chemical processes are a relevant task for modern natural sciences and have great practical value.
Meet Oza
Pandit Deendayal Petroleum University, India
Title: Experimental study of combined electrolytes for electrochemical deburring process

Biography:
Meet Oza is pursuing his graduation in Mechanical Engineering from Pandit Deendayal Petroleum University, Gandhinagar, Gujarat, India. He had participated in an industrial innovation competition organized by Larsen & Toubro (L&T) Technological Services, in which he secured a position in top 20 participants out of 7000, at the national level.
Abstract:
Statement of the Problem: Electrochemical deburring (ECD) appears to be very promising in the field of precision manufacturing. High precision and machining rates of this micro-machining technique has led to a wide variety of industrial applications, especially in mass production cycles. The study in this paper focuses on the application of combination of different electrolytes and control over the variation of its composition during ECD operations. In this study, various combinations of sodium chloride and sodium nitrate solutions are used as electrolytes and the performance of ECD has been evaluated.
Methodology & Theoretical Orientation: Testing solutions, with various percentage combinations were prepared in laboratory for various combinations of electrolytes, sodium chloride and sodium nitrate, with individual concentrations providing maximum machining rates. Conductivity was measured for each sample and recorded. Standard conductivity versus composition charts and equations were prepared corresponding to the measurements. Experimental trials were conducted on the available setup. The material removal rates and current densities were calculated and compared.
Conclusion & Significance: The measured values of conductivity for these combinations show a growth with respective increment in sodium chloride proportions. The interpolation models obtained from the plots can be utilized in industrial ECD operations to control and manipulate concentration of electrolytes. The MRR and current densities also are proportionate to the percentage of sodium chloride in the solution. Hence, it can be concluded that the effect of sodium chloride in the combination is more dominant. At the same time more stray cuts are observed with higher NaCl values.
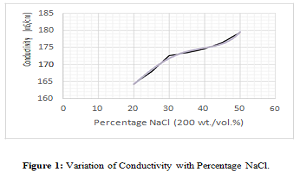
Harsh Thakkar
Pandit Deendayal Petroleum University, India
Title: Experimental investigations of ECD process parameters

Biography:
Harsh Thakkar is pursuing graduation in Mechanical Engineering at Pandit Deendayal Petroleum University, Gandhinagar, Gujarat, India. He has been working on topic of Electrochemical Deburring for the past two and half years. He was the Member and Vice President of Team INDEAGLES who participated in SUPRA SAEINDIA, in which the team secured 56th rank for the first time out of 250 teams at national level.
Abstract:
Statement of the Problem: A rapid increment in demand of highly accurate, repeatable finishing process for high value added applications, like aerospace and automotive, has led to the development of technologies like electrochemical deburring (ECD). Thus, it is of utmost importance to evaluate and optimize the performance of ECD process. This study is focused on evaluation of ECD process by comparison of material removal rates and maximum current obtained by variation in process parameters like machining time, electrolyte concentration.
Methodology & Theoretical Orientation: The paper mainly aims to evaluate the performance of electrochemical deburring process by varying certain process parameters during the deburring of internal holes. These parameters include the cycle time, electrolyte type, electrolyte concentrations. The results are obtained by a comparative study of several experimentations and characterization of the deburred area.
Conclusion & Significance: The analysis of ECD process helps to obtain the optimum value of parameters that is essential to increase the productivity of industries that require deburring of components. Deburring of internal geometries can be done by various processes like hand deburring, honing, abrasive jet machining etc. but all these processes do not ascertain specific spatial control over the deburred area, which is possible with electrochemical deburring. Hence, it is important to evaluate the performance of ECD and also to check the economic viability of the process.